Synthesis of powders in an oscillating process gas: Reactor design and application for catalytic active materials (Poster)
Homogeneous process conditions are essential for the production of oxide powders, such as advanced ceramics or catalysts. Another advantage is continuous processing. Glatt Powder Synthesis represents a further development of spray drying [1-3]. By using an oscillating process gas stream, special thermodynamic conditions are created within a tubular reactor. The modulation in the pressure ratios caused by the pulsation as well as the freely adjustable amplitudes of the pulsation intensify the mass and heat flow by a factor of 2-5. A secondary breakdown caused by the oscillating process gas stream, the Glatt Powder Synthesis is not limited to the generation of droplets and the properties of the spray solution compared to conventional spray drying methods. The powders obtained show a high homogeneity of their properties due to the homogeneous treatment in the reactor.
The Glatt Powder Synthesis is not only suitable to produce powders but also for the coating of materials. Starting from suspensions, drying, coating and calcination can be done in one step by a core-shell process.
Besides the presentation of the technology, the fabrication of photocatalysts as well as oxide materials for the generation of hydrogen will be exemplified.
Authors:
T. Jähnert, J. Buchheim, V. Drescher, Glatt Ingenieurtechnik GmbH, Weimar
M. Franke, Friedrich Schiller University Jena, Jena, Germany
B. Jäger, A. Bekisch, Fraunhofer IKTS, Hermsdorf, Germany
Materials and experimental procedure
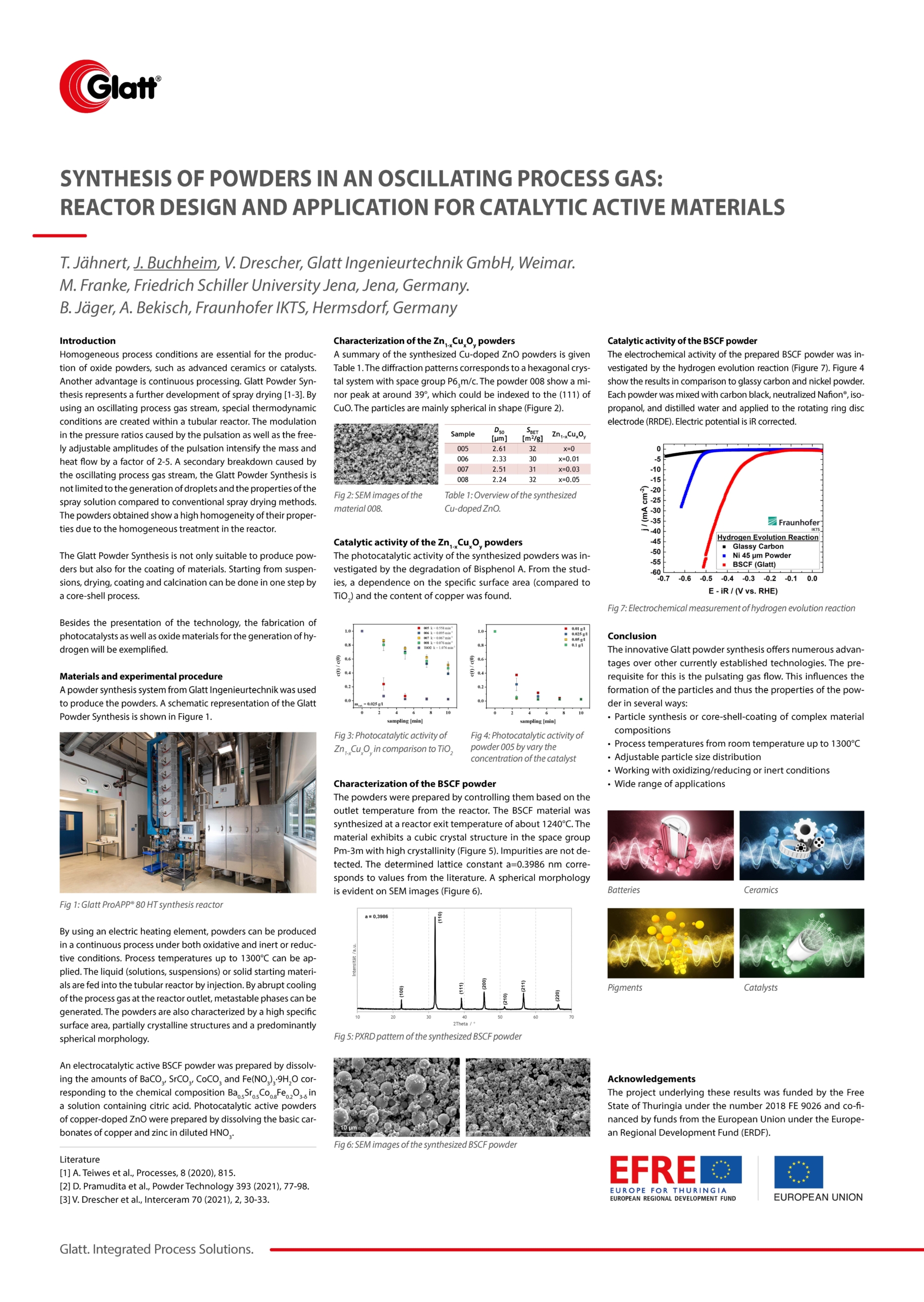
A powder synthesis system from Glatt Ingenieurtechnik was used to produce the powders. A schematic representation of the Glatt Powder Synthesis is shown in Figure 1.
By using an electric heating element, powders can be produced in a continuous process under both oxidative and inert or reductive conditions. Process temperatures up to 1300°C can be applied. The liquid (solutions, suspensions) or solid starting materials are fed into the tubular reactor by injection. By abrupt cooling of the process gas at the reactor outlet, metastable phases can be generated. The powders are also characterized by a high specific surface area, partially crystalline structures and a predominantly spherical morphology.
An electrocatalytic active BSCF powder was prepared by dissolving the amounts of BaCO3, SrCO3, CoCO3 and Fe(NO3)3∙9H2O corresponding to the chemical composition Ba0.5Sr0.5Co0.8Fe0.2O3-δ in a solution containing citric acid. Photocatalytic active powders of copper-doped ZnO were prepared by dissolving the basic carbonates of copper and zinc in diluted HNO3.
Characterization of the Zn1-xCuxOy powders
A summary of the synthesized Cu-doped ZnO powders is given Table 1. The diffraction patterns corresponds to a hexagonal crystal system with space group P63m/c. The powder 008 show a minor peak at around 39°, which could be indexed to the (111) of CuO. The particles are mainly spherical in shape (Figure 2).
Catalytic activity of the Zn1-xCuxOy powders
The photocatalytic activity of the synthesized powders was investigated by the degradation of Bisphenol A. From the studies, a dependence on the specific surface area (compared to TiO2) and the content of copper was found.
Characterization of the BSCF powder
The powders were prepared by controlling them based on the outlet temperature from the reactor. The BSCF material was synthesized at a reactor exit temperature of about 1240°C. The material exhibits a cubic crystal structure in the space group Pm-3m with high crystallinity (Figure 5). Impurities are not detected. The determined lattice constant a=0.3986 nm corresponds to values from the literature. A spherical morphology is evident on SEM images (Figure 6).
Catalytic activity of the BSCF powder
The electrochemical activity of the prepared BSCF powder was investigated by the hydrogen evolution reaction (Figure 7). Figure 4 show the results in comparison to glassy carbon and nickel powder. Each powder was mixed with carbon black, neutralized Nafion®, isopropanol, and distilled water and applied to the rotating ring disc electrode (RRDE). Electric potential is iR corrected.
Conclusion
The innovative Glatt powder synthesis offers numerous advantages over other currently established technologies. The prerequisite for this is the pulsating gas flow. This influences the formation of the particles and thus the properties of the powder in several ways:
- Particle synthesis or core-shell-coating of complex material compositions
- Process temperatures from room temperature up to 1300°C
- Adjustable particle size distribution
- Working with oxidizing/reducing or inert conditions
- Wide range of applications
Acknowledgements
The project underlying these results was funded by the Free State of Thuringia under the number 2018 FE 9026 and co-financed by funds from the European Union under the European Regional Development Fund (ERDF).
Literature
[1] A. Teiwes et al., Processes, 8 (2020), 815.
[2] D. Pramudita et al., Powder Technology 393 (2021), 77-98.
[3] V. Drescher et al., Interceram 70 (2021), 2, 30-33.

 Copyright: Glatt
Copyright: Glatt Copyright: Springer Fachmedien Wiesbaden GmbH
Copyright: Springer Fachmedien Wiesbaden GmbH